Multi-centre study in the Netherlands found that 50% of 1411 patients treated with a range of drug regimens did not require a head covering whilst scalp cooling[1]
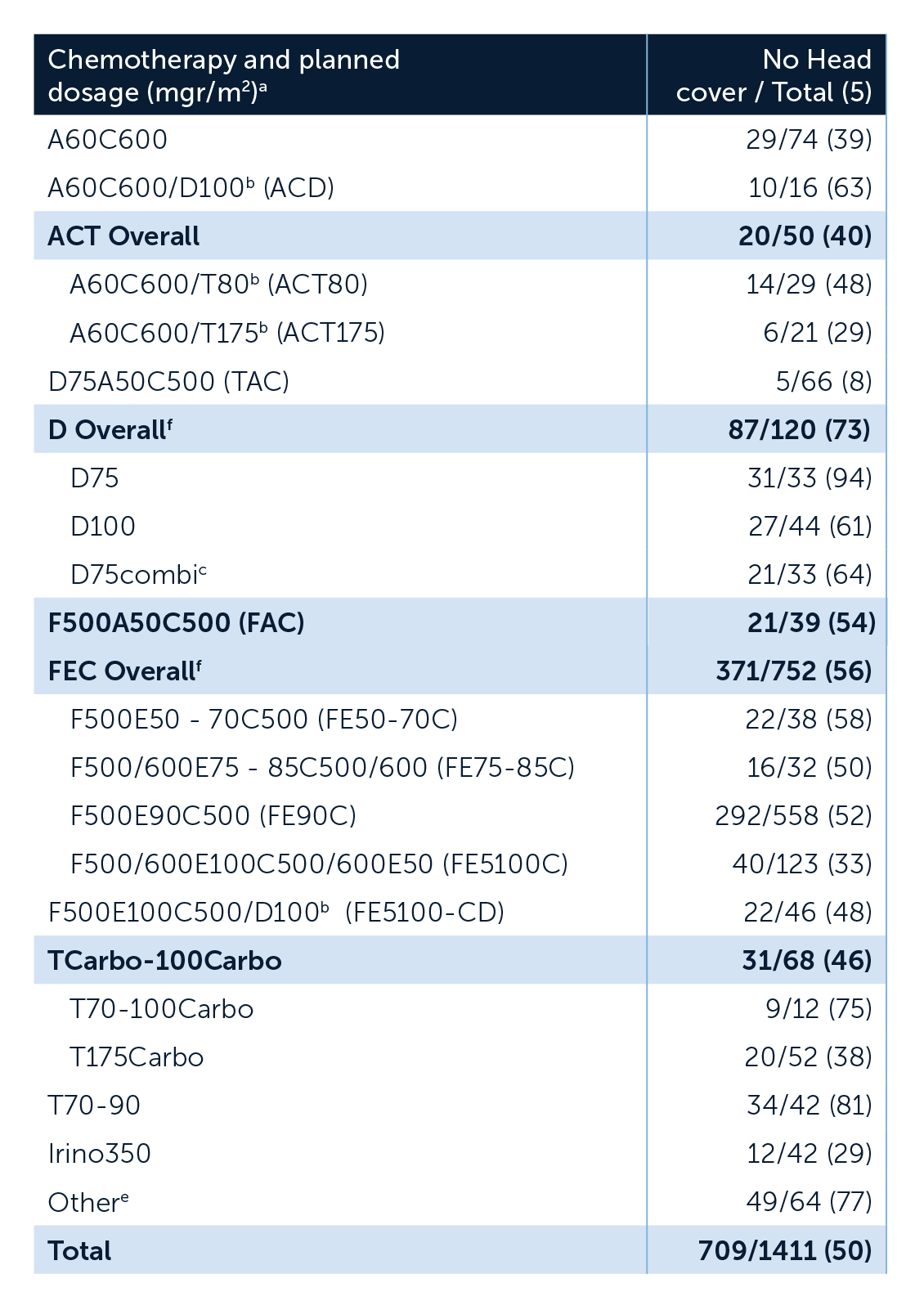
a Dosage other/missing, but included in multivariate analyses: TAC n = 1, FAC n = 4, FECD n = 6, n = 2, Irino n = 5.
b Sequential scheme.
c D combo: I) combined with Cyclophosphamide, Capecitabine, Carboplatin, Gemcitabine, Methotrexaat, Mycet or Xeloda.
d According to Dutch Guidelines.
e Other:< 10patients had a particular regimen with a specific dosage.
f Including also other dosages than specified in this table.
A: doxorubicine; Carbo: carboplatin; C: cyclophosphamide; D: docetaxel; E: epirubicine; F: 5-fluorouracil; Irino: irinotecan T: paclitaxel.
All 3 weekly schemes, with exception of /T80 and T70-90.
The Dutch Scalp Cooling Registry data showed that when scalp cooling no head cover was used by 50% of patients who received chemotherapy regimens that normally cause severe CIA. ‘This outcome is not very optimal; however, when a patient has a 50% chance to keep their hair during chemotherapy, it will be an incentive for many patients to choose scalp cooling.’[1]
Limitations – Doses of chemotherapy drugs were generally higher than in previous studies and several new drugs were evaluated giving a low success rate. The Dutch Scalp Cooling Registry started in 2006 and continues to expand and be updated.
Hair preservation was oncologist graded at 53 of 95 successful patients in the cooling group and 0 of 47 successful patients in the control group.[2]
aDefined as eligible and randomized participants in the interim analysis who underwent at least 1 cycle of chemotherapy.
bThe seventh study site recently opened and had no patients included in the interim analysis.
Sites with more experience related to scalp cooling had improved outcomes of hair preservation or patient satisfaction with hair preservation, which demonstrates the importance of structured implementation and training.[2]
The trial was stopped early for superiority and there was no effect on measures of quality of life.[2]
*ASCO update - It was shown that the difference in success rates between the cooling and non-cooling groups had improved to 53.1%. Within the cooling group, it was shown that the success rates for taxanes and anthracyclines were 63% and 24.1%. Both of which are above the values in the post hoc analysis (Nangia, et al. ASCO Poster 2017).
Investment and commitment across the full oncology team will ensure the best possible scalp cooling outcomes and a positive patient experience
Sites with more experience related to scalp cooling had improved outcomes of hair preservation or patient satisfaction with hair preservation, which demonstrates the importance of structured implementation and training.2
Summary of Success in Hair Preservation Assessed by Clinician in Modified Intent-to-Treat Populationa
aThe modified intent-to-treat population was defined as eligible and randomized participants in the interim analysis who underwent at least 1 cycle of chemotherapy
bHair preservation was graded according to Common Terminology Criteria for Adverse Events version 4.0. Grade 0 or 1 alopecia was considered a success; grade 2 alopecia was considered failure
cSuccess rate difference was 50.5% (95%CI, 40.5%-60.6%; Fisher exact test, 1-tailed P < .001)
dThe seventh study site recently opened and had no patients included in the interim analysis
eCochran-Mantel-Haenszel test, P < .001
The SCALP Study showed variability in the rate of hair preservation by site. This may be due to:
The fit of the cap is key to successful hair retention with scalp cooling. There is a learning curve with the use of the device; with repeated use, clinicians become more skilled at ensuring a tight fit and there is a higher likelihood of hair retention.[2]
With more experience, it is easier to internally train oncology teams on cap fitting. Clinical teams with higher levels of experience are also better placed to support the patient through the more challenging stages of scalp cooling.
This demonstrates the importance of structured implementation of scalp cooling, training, and continued commitment to protocols and patient experience. There will be an inevitable learning curve, but continuous dedication to improvement will help maintain enthusiasm for scalp cooling within a clinical team.
